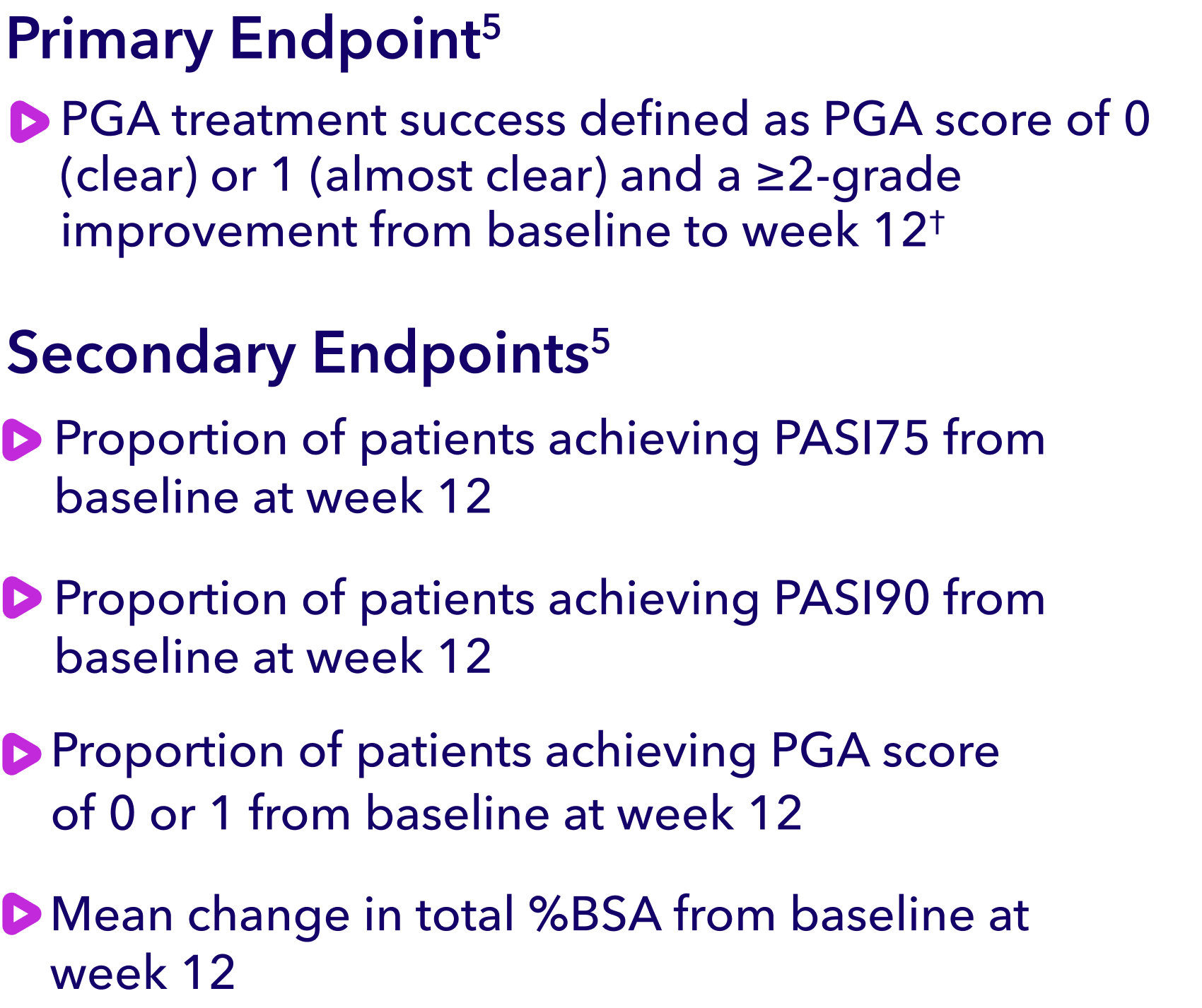
Safe and well
tolerated even on
sensitive skin areas
VTAMA cream on affected areas.3-5
Sign up today to get the latest around VTAMA (tapinarof) cream, 1%, and other helpful tips and information.
*Required field
Send your patients a link to get started with the MyVTAMATM Savings Program
*Required field
Send your patients a link to a video that will show them how to apply VTAMA cream!
*Required field
When you visit any website, it may store or retrieve information on your browser, mostly in the form of cookies. This information might be about you, your preferences or your device and is mostly used to make the site work as you expect it to. The information does not usually directly identify you, but it can give you a more personalized web experience. Because we respect your right to privacy, you can choose not to allow some types of cookies. Click on the different category headings to find out more and change our default settings. However, blocking some types of cookies may impact your experience of the site and the services we are able to offer.
 Strictly Necessary Cookies Strictly Necessary Cookies
|
Always active |
 Performance Cookies Performance Cookies
|
|
 Targeting Cookies Targeting Cookies
|

Robert, 36
Not an actual patient
Your adventurous patient
• Concerned about plaque psoriasis flare-ups limiting his active lifestyle. During a flare-up, his affected BSA can go up to 5% and his PGA score is 3 (moderate)
• Wants something to help keep his psoriasis plaques clearer so he doesn’t have to worry about them
 Previous
Previous


Emily, 51
Not an actual patient
Your active and public‑facing patient
• Works in a public-facing job and is self-conscious about her appearance when having a plaque psoriasis flare
• Has mild plaque psoriasis and is not a candidate for biologics
• Cycled through different over-the-counter steroid creams, which did not give her the results she was looking for
• Does a lot of research but relies on her dermatologist for her best options
 Previous
Previous


Carlos, 55
Not an actual patient
Your patient with sensitive skin
• Has an affected BSA of 10%, and psoriasis plaques are concentrated in sensitive areas
• Has psoriasis plaques on sensitive areas of his body, including his genitals, causing embarrassment
• Felt topical corticosteroids were not the right option for him due to his treatment area
• Wants to get back to socializing and dating without worrying about psoriasis plaques
 Previous
Previous